[ad_1]
It may be tough to find out the best manner to supply chemotherapy medicines to tumor cells. The remedies ought to ideally goal tumor cells and spare wholesome cells.
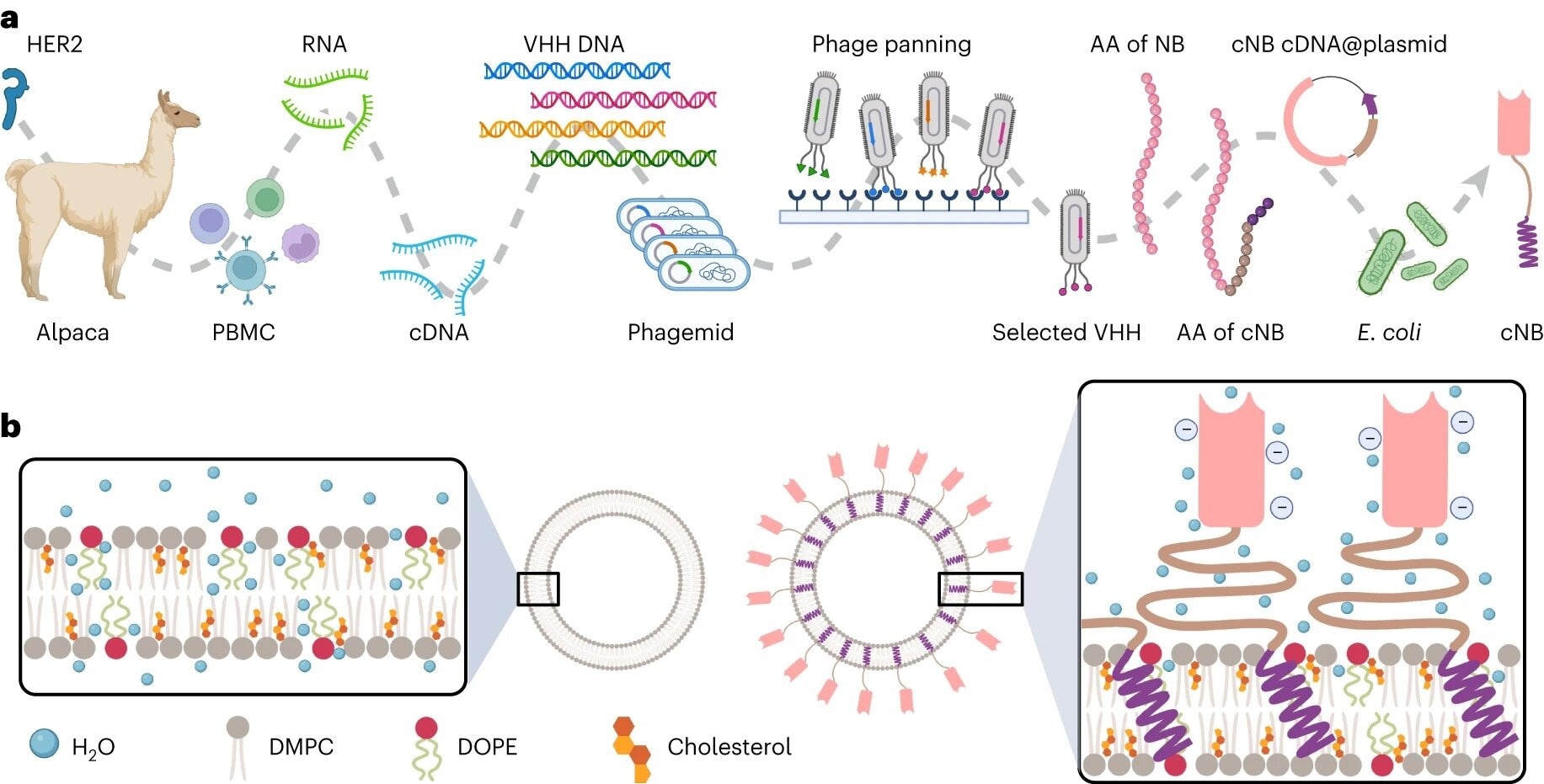
Schematic of immunoliposome preparation. a, Flowchart illustrating the manufacturing processes of cNB. Alpacas have been immunized with the HER2 extracellular area, and subsequent isolation of peripheral blood mononuclear cells (PBMCs) was carried out. Whole RNA was extracted from the PBMCs adopted by cDNA synthesis for amplifying variable heavy area of heavy chain (VHH) gene areas. The PCR merchandise have been then ligated into the phagemid vector, and E. coli cells have been remodeled with the ligated merchandise and cultured. Colonies have been recovered for the biopanning of phage displayed VHH libraries. One particular VHH was chosen and sequenced to find out its amino acid sequences. Subsequently, within the design course of amino acids encoding a hydrophilic linker (proven in yellow) and a STMD (proven in purple) have been added to the C-terminus of the NB. The corresponding cDNA was synthesized and built-in into plasmids for the expression of the cNB utilizing E. coli cells. b, Overview of biophysical transformation resulting from measurement, floor cost, lipid fluidity, membrane stiffness and thermostability between liposome and cNB-LP. AA, amino acid. Picture Credit score Nature Nanotechnology (2024). DOI: 10.1038/s41565-024-01620-6
Immunoliposomes may be the answer. They’ll effectively hook up with antigens on tumor cell surfaces by way of their surface-targeting ligands, giving tumor cells sufficient time to soak up the “poison.”
The benefits of immunoliposomes in most cancers remedy have been well-recognized all through the final 4 a long time. Nonetheless, immunoliposomal medicine haven’t but reached the market, regardless of being confirmed in laboratories since 1981.
Why? One vital obstacle is the shortage of a large-scale, low-cost, and viable manufacturing expertise. Grafting concentrating on ligands onto plain liposomes to create immunoliposomes takes roughly a half a dozen levels and may trigger issues.
A one-step manufacturing strategy for immunoliposome synthesis was just lately reported within the journal Nature Nanotechnology by Yuan Wan, an affiliate professor at Binghamton College‘s Thomas J. Watson School of Engineering and Utilized Science. It’s environmentally pleasant as a result of it doesn’t require any chemical conjugation or essential chemical reagents.
The standard manufacturing technique of immunoliposomes is comparatively complicated. It entails loads of chemical conjugation and purification. Chemical conjugation and required reagents impair the soundness and antigen binding of concentrating on ligands. The multistep course of results in payload leakage and product loss.
Yuan Wan, Affiliate Professor, Division of Biomedical Engineering, Binghamton College
Wan added, “So, immunoliposomes are much less engaging to industrial producers due to their low yield, excessive manufacturing prices, and the excessive threat of batch-to-batch variation. These shortcomings hinder business manufacturing and scientific use of immunoliposomes.”
What distinguishes Wan’s research is the usage of manufactured chimeric nanobodies with a “sticky” finish. Greater than 2,500 nanobodies could be built-in into the outside of a single 100-nanometer liposome, which is roughly 1,000 occasions smaller than a human hair.
This course of is easier, faster, and cheaper than earlier procedures, and it offers extra affect over the ultimate product. The floor nanobodies additionally create a protecting barrier across the liposome, which may cease it from being eradicated by the physique too quickly and permit it to stay in circulation for longer.
One other vital benefit is that this process doesn’t use dangerous chemical compounds. Conventional strategies regularly contain a substance often called polyethylene glycol (PEG), which might often create difficulties for sufferers, together with demise. Due to these considerations, the US Meals and Drug Administration mandates extra monitoring for medicine containing PEG.
“One thing actually attention-grabbing we discovered is that when these chimeric nanobodies are inserted into the lipid bilayer, they really enhance the rigidity and thermal stability of your entire immunoliposomes. So, the medicine packed inside can maintain up for a superb 10 months with out apparent leaks,” Wan said.
Moreover, Wan is optimistic that, with extra analysis and scientific testing, immunoliposomes may finally be produced and obtain federal approval for scientific use, provided that there are actually about 20 plain liposomal medicine in use.
Wan concluded, “We’re additionally engaged on growing new chimeric nanobodies to ramp up manufacturing by at the least 30 occasions. It is going to make the manufacturing value of those chimeric nanobodies a lot decrease.”
Journal Reference:
Rahman, M. M., et. al. (2024) Chimeric nanobody-decorated liposomes by self-assembly. Nature Nanotechnology. doi:10.1038/s41565-024-01620-6.
Supply: https://www.binghamton.edu/
[ad_2]
Supply hyperlink




