[ad_1]
Mar 14, 2024
(Nanowerk Information) Protein-based medicine should be transported into cells in a means that forestalls their fast degradation. A brand new method is meant to make sure that they continue to be intact solely in sure cells, equivalent to most cancers cells. Within the journal Angewandte Chemie (“Selective Intracellular Supply of Antibodies in Most cancers Cells with Nanocarriers Sensing Endo/Lysosomal Enzymatic Exercise”), a Japanese analysis group has launched a nanocarrier that may “escape” from endosomes earlier than its cargo is destroyed there. This means to flee is just triggered throughout the endosomes of sure tumor cells.
Selective Intracellular Supply of Antibodies in Most cancers Cells with Nanocarriers Sensing Endo/Lysosomal Enzymatic Exercise. (Picture: Wiley)
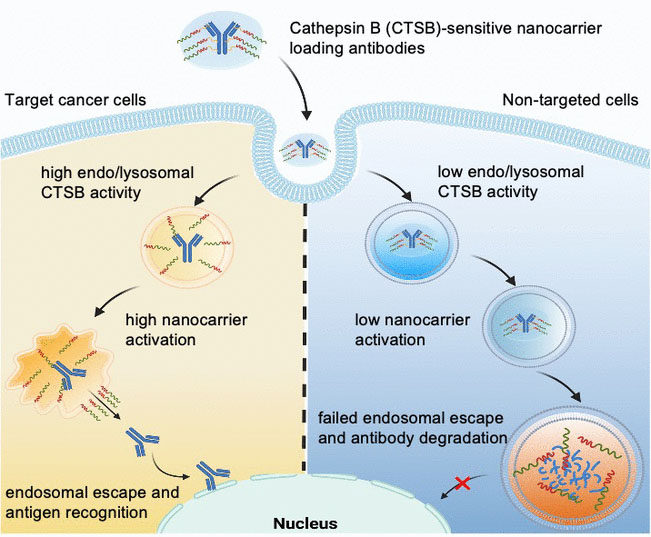
The uptake of nanocarriers into cells happens by endocytosis: when a nanocarrier lands on the cell floor, the cell membrane folds in and encloses it in a bubble, referred to as an endosome, which then drifts into the cell inside. In its late section, the endosome merges with lysosomes that comprise enzymes, forming an endolysosome. Inside this construction, the enzymes break down each materials from the physique and international materials. A protein-based drug can solely turn into lively if it “escapes” the endolysosome earlier than being damaged down. This is called “endosomal escape”. Some nanocarriers can open the endo/lysosomal membrane and thus have endosomal escape means.
Led by Kazunori Kataoka and Horacio Cabral, the group goals to take this a step additional by producing nanocarriers for which endosomal escape is just triggered once they enter very particular cells, equivalent to tumor cells. This may defend wholesome cells. The researchers exploit the truth that various kinds of cells have very completely different endolysomomal enzyme actions. For instance, the exercise of the protease cathepsin B (CTSB) is very excessive in most cancers cells.
With using particular fluorescence probe molecules, the group from The College of Tokyo and the Kawasaki Institute of Industrial Promotion initially studied CTSB exercise and protein degradation in endosomes. They decided that in most cancers cells with extremely acidic endosomes, CTSB exercise is already very excessive of their early section—considerably earlier than protein degradation ramps up. The researchers make the most of this time window through the use of nanocarriers whose endosomal escape means is triggered by the CTSB in most cancers cells.
The group constructed poly(ethylene glycol)-based nanocarriers with diaminoethane teams able to “tearing open” endo/lysosomal membranes. Utilizing a linker, they then hooked up antibodies to behave as a mannequin for a protein drug. The nanocarrier shields the “tearing instruments” in order that they’re initially inactive. The linker is designed to be break up by the CTSB within the endolysosomes. This separates the cargo from the provider, activating the tearing instruments. They open the endo/lysosomal membrane and launch intact antibodies into the cell inside—however solely in tumor cells which have elevated endosomal CTSB exercise.
This methodology may symbolize a brand new technique for the cell-specific launch of medicine via stimulus-responsive nanocarriers with managed endosomal escape.
[ad_2]
Supply hyperlink




