[ad_1]
Mar 05, 2024
(Nanowerk Information) Catalysts unlock pathways for chemical reactions to unfold at quicker and extra environment friendly charges, and the event of latest catalytic applied sciences is a crucial a part of the inexperienced vitality transition.
The Rice College lab of nanotechnology pioneer Naomi Halas has uncovered a transformative strategy to harnessing the catalytic energy of aluminum nanoparticles by annealing them in varied fuel atmospheres at excessive temperatures.
In keeping with a research revealed within the Proceedings of the Nationwide Academy of Sciences (“Tailoring the aluminum nanocrystal floor oxide for all-aluminum-based antenna-reactor plasmonic photocatalysts”), Rice researchers and collaborators confirmed that altering the construction of the oxide layer that coats the particles modifies their catalytic properties, making them a flexible instrument that may be tailor-made to go well with the wants of various contexts of use from the manufacturing of sustainable fuels to water-based reactions.
“Aluminum is an earth-abundant steel utilized in many structural and technological purposes,” stated Aaron Bayles, a Rice doctoral alum who’s a lead writer on the paper. “All aluminum is coated with a floor oxide, and till now we didn’t know what the construction of this native oxide layer on the nanoparticles was. This has been a limiting issue stopping the widespread utility of aluminum nanoparticles.”
Aluminum nanoparticles take up and scatter gentle with exceptional effectivity resulting from floor plasmon resonance, a phenomenon that describes the collective oscillation of electrons on the steel floor in response to gentle of particular wavelengths. Like different plasmonic nanoparticles, the aluminum nanocrystal core can operate as a nanoscale optical antenna, making it a promising catalyst for light-based reactions.
“Nearly each chemical, each plastic that we use on a day-to-day foundation, got here from a catalytic course of, and lots of of those catalytic processes depend on treasured metals like platinum, rhodium, ruthenium and others,” Bayles stated.
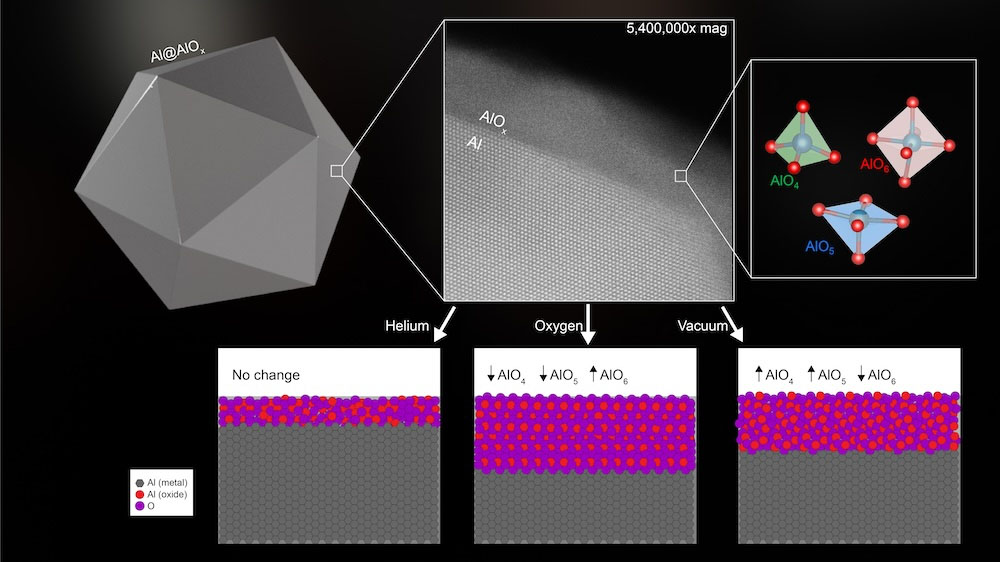
The highest half of the picture reveals a schematic illustration of an aluminum oxide nanoparticle (left), a microscope picture of the oxide layer coating the floor of the nanoparticle (center) and a illustration of the totally different items within the alumina layer composition (proper). The underside half illustrates the impact of annealing on the oxide construction, which adjustments the thickness and association of the atoms, leading to modified optical and floor chemical properties of the aluminum nanoparticles. (Picture courtesy of Aaron Bayles/Rice College)
“Our final purpose is to revolutionize catalysis, making it extra accessible, environment friendly and environmentally pleasant,” stated Halas, who’s a College Professor, Rice’s highest tutorial rank. “By harnessing the potential of plasmonic photocatalysis, we’re paving the best way for a brighter, extra sustainable future.”
The Halas group has been growing aluminum nanoparticles for plasmonic photocatalysis reactions equivalent to decomposition of harmful chemical warfare brokers and environment friendly manufacturing of commodity chemical compounds. The newly uncovered means to switch the floor oxides on aluminum nanoparticles additional will increase their versatility to be used as catalysts to effectively convert gentle into chemical vitality.
“For those who’re doing a catalytic response, the molecules of the substance you’re trying to remodel will work together with the aluminum oxide layer relatively than with the aluminum steel core, however that metallic nanocrystal core is uniquely in a position to take up gentle very effectively and convert it into vitality, whereas the oxide layer fulfills the position of a reactor, transferring that vitality to reactant molecules,” Bayles stated.
The properties of the nanoparticles’ oxide coating decide how they work together with different molecules or supplies. The research elucidates the construction of this native oxide layer on aluminum nanoparticles and reveals that easy thermal therapies ⎯ i.e. heating the particles to temperatures of as much as 500 levels Celsius (932 Fahrenheit) in numerous gasses ⎯ can change its construction.
“The crystalline part, intraparticle pressure and defect density can all be modified by this easy strategy,” Bayles stated. “Initially, I used to be satisfied that the thermal therapies did nothing, however the outcomes shocked me.”
One of many results of the thermal therapies was to make the aluminum nanoparticles higher at facilitating the conversion of carbon dioxide into carbon monoxide and water.
“Altering the alumina layer on this method impacts its catalytic properties, notably for light-driven carbon dioxide discount, which suggests the nanoparticles could possibly be helpful for producing sustainable fuels,” stated Bayles, who’s now a postdoctoral researcher on the Nationwide Renewable Power Laboratory.
Bayles added that the flexibility “to make use of plentiful aluminum rather than treasured metals could possibly be vastly impactful to fight local weather change and opens the best way for different supplies to be equally enhanced.”
“It was comparatively simple to do these therapies and get massive adjustments in catalytic conduct, which is shocking as a result of aluminum oxide is famously not reactive ⎯ it is rather secure,” Bayles stated. “So for one thing that may be a little bit extra reactive ⎯ like titanium oxide or copper oxide ⎯ you may see even larger results.”
[ad_2]
Supply hyperlink




