[ad_1]
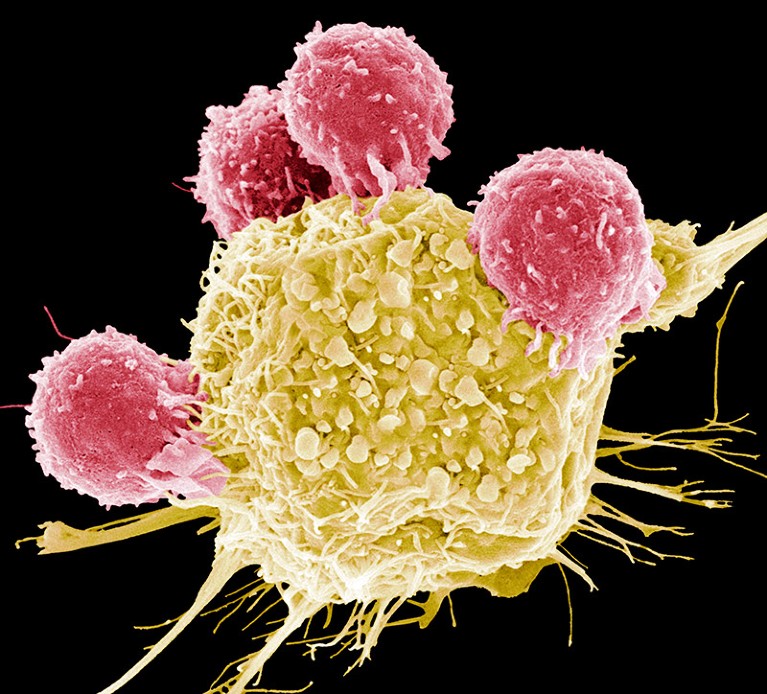
T cells (pink) assault a most cancers cell (yellow) on this scanning electron micrograph picture.Credit score: Steve Gschmeissner/SPL
A small Indian biotechnology firm is producing a home-grown model of a cutting-edge most cancers remedy often called chimeric antigen receptor (CAR) T-cell remedy that was pioneered in america. CAR-T therapies are used primarily to deal with blood cancers and have burgeoned prior to now few years. The Indian CAR-T remedy prices one-tenth that of comparable business merchandise accessible globally.
A single remedy of NexCAR19, manufactured by Mumbai-based ImmunoACT, prices between US$30,000 and $40,000. The primary CAR-T remedy was authorized in america in 2017, and business CAR-T therapies presently value between $370,000 and $530,000, not together with hospital charges and medicines to deal with unintended effects. These therapies have additionally proven promise in treating autoimmune ailments and mind most cancers.
India’s drug regulator authorized NexCAR19 for therapeutic use in India in October. By December, ImmunoACT was administering the remedy to paying sufferers, and it’s now treating some two-dozen individuals a month in hospitals throughout the nation.
“It’s a dream come true,” says Alka Dwivedi, an immunologist who helped to develop NexCAR19 and is now on the US Nationwide Most cancers Institute (NCI) in Bethesda, Maryland. Her voice turns into tender as she describes seeing the primary affected person’s most cancers go into remission. These are individuals for whom all different therapies have failed, says Dwivedi. “They’re getting cured.”
“It’s very optimistic information,” says Renato Cunha, a haematologist on the Grupo Oncoclínicas in São Paulo, Brazil. He says the Indian product might pave the way in which for making superior mobile therapies accessible to different low- and middle-income international locations. “Hope is the phrase that involves thoughts.”
The product can be a actuality examine for researchers in high-income international locations, says Terry Fry, an immunologist and paediatric oncologist on the College of Colorado Anschutz Medical Campus in Denver, who has suggested the researchers concerned in establishing ImmunoACT. “It lights a bit of fireplace below all of us to have a look at the price of making CAR-T cells, even in locations like america.”
Super want
CAR-T remedy includes taking somebody’s blood and isolating immune elements often called T cells. These are genetically modified within the laboratory to specific a receptor, often called a CAR, on their floor. This helps the immune cells to seek out and kill most cancers cells. The engineered cells are then mass-produced and infused again into the affected person, in whom they proliferate and get to work.
The race to supercharge cancer-fighting T cells
Information on demand for these therapies in India are restricted, however one research taking a look at a particular type of leukaemia discovered that as much as 15 individuals in 100,000 are identified with the illness, half of whom relapse inside two years of receiving remedy, reminiscent of chemotherapy, and who subsequently select palliative care1. There’s a “super affected person want”, says Nirali Shah, a paediatric oncologist on the NCI, who can be an educational collaborator of the researchers at ImmunoACT.
NexCAR19 is just like its US counterparts, but distinct in key methods. Like 4 of the six CAR-T therapies authorized by the US Meals and Drug Administration (FDA), it’s designed to focus on CD19, a marker discovered on B-cell cancers2. Nevertheless, in current business therapies, the antibody fragment on the finish of a CAR is often from mice, which limits its sturdiness as a result of the immune system acknowledges it as overseas and finally eliminates it. Due to this fact, in NexCAR19, Dwivedi and her colleagues added human proteins to the mouse antibody suggestions.
Lab research confirmed that the ‘humanized’ CAR had comparable antitumour exercise to a mouse-derived one and induced the manufacturing of decrease ranges of proteins referred to as cytokines2. That is necessary, as a result of some individuals with most cancers who obtain CAR-T remedy expertise an excessive inflammatory response often called cytokine-release syndrome, which could be life-threatening.
Trial knowledge
Early-stage scientific trials for NexCAR19 in adults with completely different types of lymphoma and leukaemia, confirmed that in 19 of the 33 individuals who acquired the remedy, the tumours had utterly disappeared on the one-month follow-up3. The tumours in one other 4 individuals had shrunk by half — attaining an general response price of 70%. Trial members will probably be adopted for no less than 5 years.
“Whether or not this may maintain or not is one thing solely time will inform,” says Hasmukh Jain, a medical oncologist at Tata Memorial Centre in Mumbai, who led the trials.
Natasha Kekre, a haematologist on the Ottawa Hospital, factors out that the outcomes are based mostly on a small variety of members with a spread of blood cancers, which makes it troublesome to evaluate the remedy’s efficacy for particular cancers.
Solely two of the members skilled extra extreme types of cytokine-release syndrome, and none had neurotoxicities, one other frequent however momentary aspect impact of CAR-T remedy.
The security profile is healthier than that of among the FDA-approved CAR-T therapies, says Kekre. This might be associated to the product, in addition to to years of the scientific and medical group studying higher look after sufferers, she says.
Humanizing the CAR in all probability contributed to the remedy’s optimistic security profile, says Rahul Purwar, an immunologist on the Indian Institute of Expertise Bombay, and founding father of ImmunoACT. However others say that hyperlink has but to be established.
Fry says the setting and sort of affected person handled in India might additionally have an effect on the outcomes. “The toxicity profile of CAR-T cells is pushed by a number of different affected person elements.”

A member of the ImmunoACT workforce making ready the NexCAR19 most cancers remedy.Credit score: ImmunoACT
Slashing prices
Though the remedy’s price ticket remains to be excessive for a lot of Indians, whose annual gross nationwide revenue per capita is lower than $2,500, NexCAR19’s value gives hope that CAR-T remedy could be made extra cheaply in different international locations and contexts. To slash prices, the workforce developed, examined and manufactured the product totally in India, the place labour is cheaper than in high-income international locations.
To introduce CARs to T cells, researchers sometimes use lentiviruses, that are costly. Buying sufficient lentiviral vector for a trial of fifty individuals can value as much as US$800,000 in america, says Steven Highfill, an immunologist on the US Nationwide Institutes of Well being Scientific Heart in Bethesda, who has suggested the Indian workforce. Scientists at ImmunoACT make this gene-delivery car themselves.
The Indian workforce additionally discovered a less expensive strategy to mass-produce the engineered cells, avoiding the necessity for costly automated equipment, says Highfill.
Sufferers’ prices are additional diminished by the remedy’s improved security profile in contrast with among the different FDA-approved merchandise, Purwar says. This meant that the majority sufferers didn’t have to spend time in intensive-care models.
Purwar hopes to additional lower prices, together with by scaling up manufacturing. ImmunoACT is planning to export the remedy to Mexico, and to develop new merchandise, together with a remedy for one more type of blood most cancers often called a number of myeloma.
However ImmunoACT faces competitors. A number of different Indian firms have launched native CAR-T trials, together with Immuneel Therapeutics in Bengaluru, which has licensed expertise developed by Spanish teachers.
[ad_2]
Supply hyperlink




